Results from laboratory trials in France and Thailand as well as eld trials in Vietnam indicate
a promising prophylactic or therapeutic application during certain critical disease outbreaks, subject to specific conditions.
Today, the challenge faced By the aquaculture industry is to maximize yield production to meet supply demands. One approach to reach this objective is to adopt a super intensive production system However, super intensive systems may ultimately lead to diseases outbreaks, introduction and spread of new pathogens. This pathogenic pressure significantly impacts the economics of farming. One solution has been the use of antibiotics to combat disease outbreaks but the massive use of chemicals in aquaculture nowadays raises public health concerns with antibiotic resistance and adverse effects on the environment.
Active research is ongoing to explore alternatives to antibiotic treatments. This article reports on research to evaluate the potential of a natural phytogenic based on specifically selected plant extracts (commercial name “A-Live”, Mix Science, France) to control a broad spectrum of pathogens in aquaculture systems, first at a laboratory scale, followed with field trials. The antimicrobial effects of the phytogenic was investigated both in vitro and in vivo.
Evaluation of potential
In vitro, minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC), of the product, respectively, were determined using microdilution methods against a wide range of pathogens from seawater and freshwater aquaculture systems. The MICs were compared to natural extracts known to have high antimicrobial potential such as citral, isolated from citrus oil, eugenol, from clove oil and carvacrol, from oregano and thyme oils. Finally, in order to evaluate the real potential of this product as an antibiotic alternative, MIC and MBC were compared to common antibiotics used in aquaculture (oxytetracycline, erythromycin and enrofloxacin).
Based on these preliminary studies, the product was then applied in challenge trials where the targeted species were either freshwater fish or marine shrimp.
Laboratory to farm trials
The in vivo laboratory scale trials for Nile tilapia Oreochromis niloticus were carried out in indoor 100 L tanks (in Ictyopharma, France). Here an independent recirculation system was used where each system was equipped with a mechanical filter and a bio-filter as well as degasing columns and ultra violet lamps. Temperature, salinity, and oxygen levels were kept within the following range: temperature 27-29°C; salinity 0 ppt and oxygen 5-7 ppm.
Tilapia juveniles of approximately 15 ±0.4 g were used. Before challenge, fish were fed for a period of 28 days with the experimental diets (with the phytogenic dose of 1 kg/tonne of feed). After 28 days, experimental infection was performed by immersion. The immersion was performed in static conditions in the rearing tanks of the animals. A fixed volume of overnight Streptococcus agalactiae culture at approximately 1×109 CFU/mL was added to each rearing tank. The sh remained in the static condition with air supply for 1 hour after which the water recirculation was turned back on. After exposure, fish remained fed with the experimental diets (with the phytogenic dose of phytogenic of 1 kg/tonne of feed) during a 21 day observation period post challenge.
A similar protocol was used for the white shrimp Litopenaeus vannamei held in indoor 115 L glass aquaria in the Prince of Songkla University, Thailand. Each aquarium was aerated continuously. Water was exchanged at 20% every day. Temperature, salinity, and oxygen levels were kept within the following range: temperature 27-28 0C; salinity 25 ppt and oxygen 5-6 ppm;
Shrimp post larvae (PL5 with average body weight of 0.88 g ± 0.003) were used for the feeding trial. Before challenge, shrimp post larvae were fed for a period of 21 days with the experimental diets (with the phytogenic dose of 1 kg/tonne of feed). After 21 days, shrimp post larvae were exposed to Vibrio parahaemolyticus responsible for early mortality syndrome (EMS) in some of the experimental tanks and white spot syndrome virus (WSSV) in other tanks. For bacterial challenge, a suspension of Vibrio parahaemolyticus was prepared from a 18-24 hours culture and was adjusted to reach a final concentration of approximately 106 CFU/mL of culture water.
For viral challenge, a WSSV suspension at the LD50 concentration previously determined was applied.
After exposure, shrimp post larvae continued to be fed with the experimental diets (with the phytogenic dose of 1 kg/ tonne of feed) during a 14 day observation period post challenge.
Finally, the effect of the phytogenic was tested under commercial farm conditions for both species in farms in Vietnam. The phytogenic was applied temporarily at a disease control dose of 4 kg/ tonne of feed during 14 days in tilapia farmed in outdoor cages after the emergence of a streptococcal infection. It was applied at the same dose for a duration of 35 days in shrimp farmed in outdoor ponds after the appearance of vibriosis.
A wide spectrum action
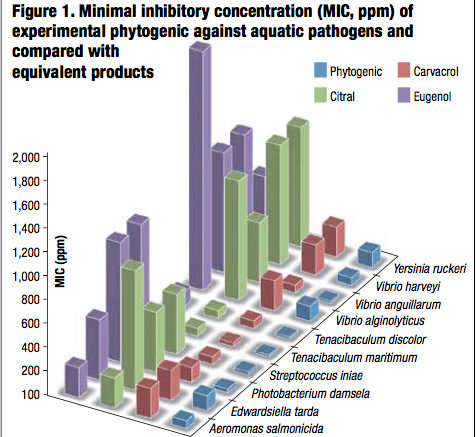
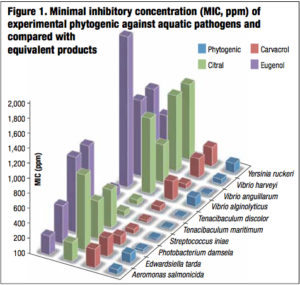
In vitro results indicated that this feed additive showed a wide spectrum bactericidal action since it exhibited high efficiency against both gram positive and gram negative bacteria (Table 1). Moreover, it showed the strongest anti-microbial activity compared to equivalent competitor products. The experimental phytogenic presented the lowest MIC from 16 to 125 ppm whereas it was from 32 to 250 ppm for carvacrol, from 64 to 1,000 ppm for citral and from 64 to 2000 ppm for eugenol (Figure 1). It also demonstrated the minimal inhibitory and bactericidal concentrations from 19 to 150 ppm and in the same order of magnitude (less than 1 log unit) for the phytogenic and the tested antibiotics. These results demonstrated the potential of the phytogenic as efficient antibiotics alternative (Table 1).
In vivo, during the research trials, there was a signicant reduction of mortality (ANOVA p<0.05) regardless of farmed species and associated pathogens (bacteria or virus) as indicated in Figure 2.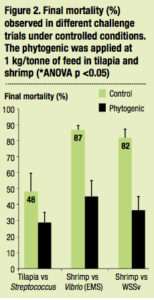
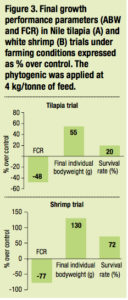
Antimicrobial effect of the phytogenic was confirmed under farming conditions where it significantly supported resistance of tilapia and shrimp (ANOVA p <0.05) when challenged with Streptococcus spp. and Vibrio spp. respectively (Figure 3).
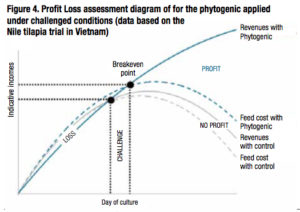
In terms of economics, under non optimal conditions and a few days after a disease outbreak, growth performance would be highly depressed. The incomes from sales of the harvest may not compensate for feed cost and the farmer would lose money. The application of the phytogenic helped to provide a good profit by maintaining significant increase in survival (20% more in sh and 72% more in shrimp) and thus a higher harvested biomass. In contrast, the harvest revenue from the control ponds did not increase and there were losses due to feed costs. This process is explained in the didactic breakeven point diagram based on the tilapia trial conducted in a fish farm in Vietnam (Figure 4).
We concluded that this new feed additive provides efficient control against a variety of pathogens and could be considered as a holistic and natural way of reducing the use of antibiotics in aquaculture systems. Trial data also showed the efficacy of the functional additive to counterbalance disease outbreaks and to maintain a reliable growth performance and farm profit. Moreover, this new phytogenic can be applied in a wide range of conditions either continuously as a prophylactic agent or during certain critical periods as a therapeutic agent. The optimal duration of application is at least 14 days before any known critical period or after the first appearance of disease symptoms.
—————————–
This article is written by Stephane Frouel, Clarisse Techer and Frederic Jozwiak,Mix Science, Avril group, Bruz, Bretagne, France